

“SULFUR TRIOXIDE.” National Center for Biotechnology Information. “Sulfur dioxide.” Wikipedia, Wikimedia Foundation, 3 Jan. The main difference between SO 2 and SO 3 is that SO 2 has two oxygen atoms bonded to a sulfur atom whereas SO 3 has three oxygen atoms bonded to a sulfur atom. SO 3 is a solid (crystalline) compound at room temperature. SO 2 is a gaseous compound at room temperature.
SO 2 and SO 3 are inorganic compounds that are named as sulfur oxides. SO 3:SO 3 is non-polar due to its geometry (trigonal planar) and the absence of a lone electron pair. SO 2: SO 2 is polar due to its geometry (angular) and the presence of a lone electron pair. SO 3: The oxidation state of sulfur in SO 3 is +6. SO 2: The oxidation state of sulfur in SO 2 is +4. SO 3: The melting point of SO 3 is about 16.9 ☌ whereas the boiling point is 45☌. SO 2: The melting point of SO 2 is about -71☌ whereas the boiling point is -10☌. SO 3:The molar mass of SO 3 is 80.057 g/mol. SO 3:SO 3 is a solid compound composed of one sulfur atom bonded to three oxygen atoms. SO 2:SO 2 is a gaseous compound composed of sulfur and oxygen atoms. Difference Between SO 2 and SO 3 Definition In addition, sulfur trioxide is an essential reagent in Sulfonation process. Therefore, control methods should be used when using sulfur trioxide for industrial sulfuric acid production. The above reaction is very rapid and exothermic. This is because sulfur trioxide is the anhydride form of sulfuric acid. However, sulfur trioxide is very important in the production of sulfuric acid in industrial scale. In its gaseous form, sulfur trioxide is an air pollutant and is a major component in acid rains. The oxidation state of sulfur in sulfur trioxide is +6. At room temperature and pressure, sulfur trioxide is a white crystalline solid compound which will fume in the air. The melting point of SO 3 is about 16.9 ☌ whereas the boiling point is 45 oC.

The molecular mass of sulfur trioxide is 80.057 g/mol. Since the oxidation state of sulfur is +4 in sulfur dioxide, it can easily be oxidized to +6 oxidation state, which allows another compound to be reduced. Sulfur dioxide is also a good reducing agent. Sulfur dioxide can be used in the production of sulfuric acid which has a number of applications in the industrial scale and the laboratory scale.

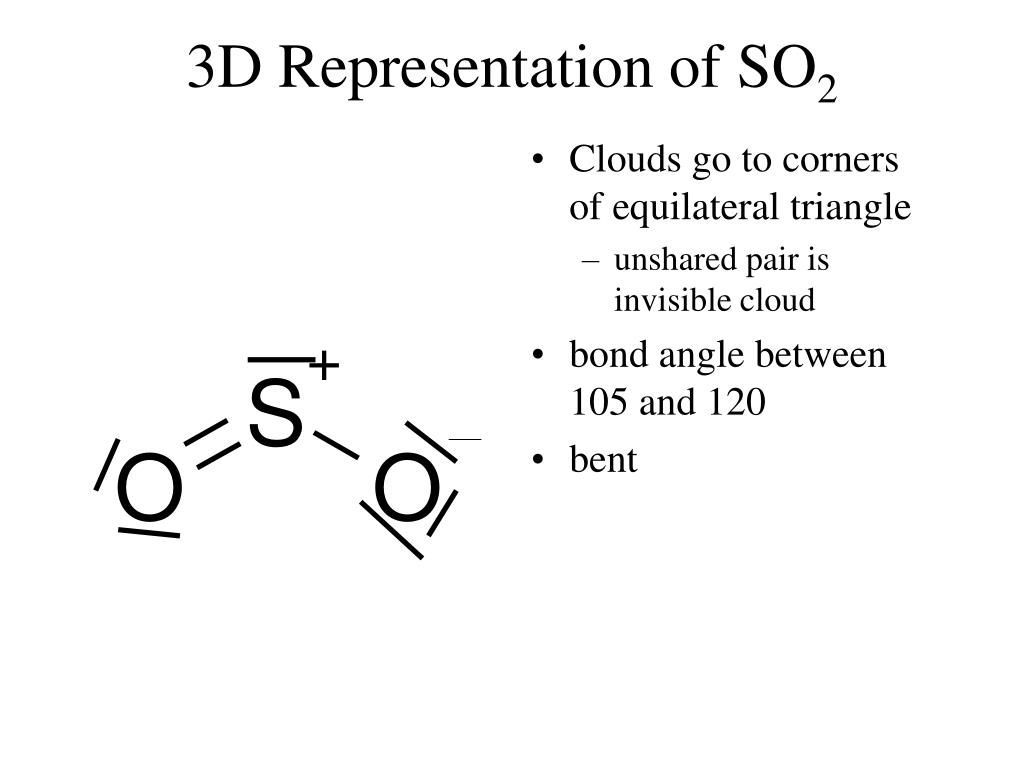
Therefore, it can be reduced to +4 oxidation state of sulfur dioxide. Here, sulfur in sulfuric acid is in the oxidation state of +6. One such example is the reaction between copper and sulfuric acid. Therefore, sulfur dioxide can also be produced by the reduction of compounds composed of sulfur atoms that are in a higher oxidation state. The oxidation state of sulfur in sulfur dioxide is +4. The melting point is about -71 oC whereas the boiling point is -10 oC. It is a colorless gas at room temperature. The molecular mass of sulfur dioxide is 64 g/mol. Hence, if there is SO 2 in the atmosphere, it will be an indication of air pollution. Sulfur dioxide is considered as a toxic gas. SO 2 is polar due to its geometry (angular) and the presence of a lone electron pair. This determines the geometry of the SO 2 molecule as angular geometry. Since sulfur element has 6 electrons in its outermost orbital after forming two double bonds with the oxygen atoms, there are 2 more electrons remaining these can act as a lone electron pair. Hence, the sulfur atom is the central atom of the compound. One oxygen atom can form a double bond with the sulfur atom. Therefore, it is composed of a sulfur atom bonded to two oxygen atoms through covalent bonds. The chemical formula of sulfur dioxide is SO 2. Sulfur dioxide is a gaseous compound composed of sulfur and oxygen atoms. Key Terms: Acid Rain, Lone Electron Pair, Oxygen, Oxidation State, Sulfur, Sulfur Dioxide, Sulfuric Acid, Sulfur Trioxide What is the Difference Between SO2 and SO3 – Definition, Chemical Structure and Properties, Sulfuric Acid Productionģ. – Definition, Chemical Structure and Properties, Oxidation State These compounds are called oxides of sulfur since they are formed from the reaction between sulfur and O 2 molecules. They have different chemical and physical properties. SO 2 stands for sulfur dioxide, and SO 3 stands for sulfur trioxide. SO 2 and SO 3 are inorganic chemical compounds formed by the combination of sulfur atoms and oxygen atoms.


 0 kommentar(er)
0 kommentar(er)
